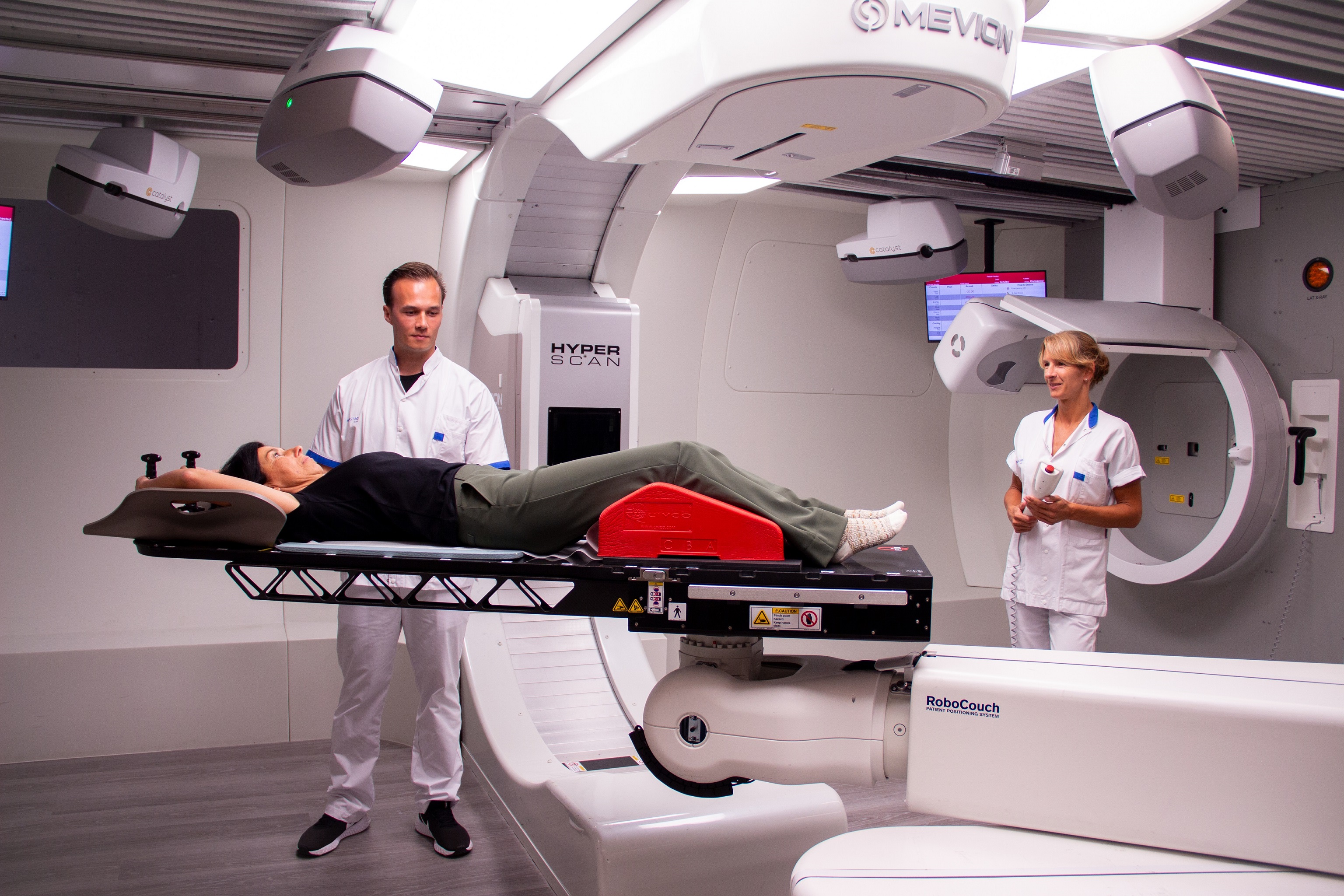
Expertise
Healthcare
Better care and cure through innovative solutions
Services, Solutions & Products
The demand for better care and cures through innovative solutions is high. We offer a wide range of services, solutions and products to help healthcare organisations and suppliers with their ambitions and objectives. Our professional project office offers all services for the development of medical (software) products. We combine the knowledge that we as ICT Group have of technology, safety and interoperability with far-reaching domain knowledge of medical practice and all relevant certifications, such as ISO 13485. As a result, we can also guide parties through intensive processes towards CE marking, FDA submission & approval and the Medical Devices Regulation (MDR).
Solutions for obstetrics
Our software solutions are designed for electronic registration of fetal and maternal parameters, aimed at optimising work and decision-making processes in the maternity department. The development of the integral solutions for obstetrics takes place in close cooperation with gynaecologists, midwives, universities and government agencies.
More information?
Do you want to work in Healthcare?
Are you a specialist in the field of Healthcare looking for a fun and challenging position? Within ICT Group we have various opportunities in this area.